Charder receives MDR certification

Charder is proud to announce that it has officially received MDR certification!
What is MDR, and why is this important news?
Definition
The Medical Device Regulation (EU Regulation No. 2017/745) replaces the previous Medical Device Directive (90/385/EEC & 93/42/EEC) governing how medical devices are designed, produced, brought to market, and tracked in Europe.
Why is MDR replacing MDD?
An important goal of MDR is to improve patient safety, and it is significantly more strict compared to MDD. A medical device needs to be safe and effective throughout its entire lifetime, and MDR places additional focus on tracking devices after they are placed on the market, and fixing problems promptly when reported.
MDR is not an easy certificate to acquire, and the number of Notified Bodies issuing MDR certificates is insufficient to meet demand.
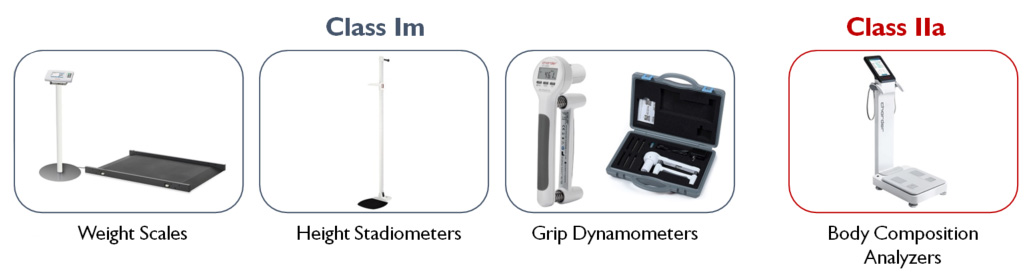
Current MDD certifications for "legacy devices" can be extended to be valid until 31 Dec 2028 at the very latest (Class Im, Class IIa), assuming manufacturers are compliant with foregoing requirements, such as establishing a Quality Management System compliant with MDR by 26 May 2024.
What this means for you
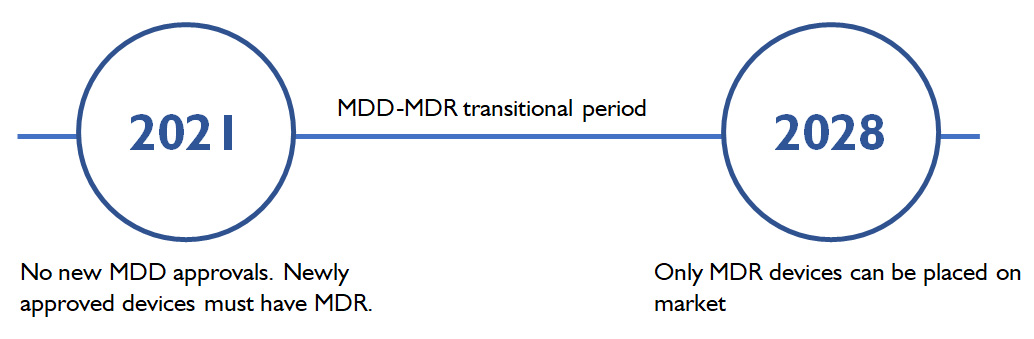
Many devices and manufacturers will not be able to remain on the market after MDD expiration, if they cannot acquire MDR. Thus, it is recommended to choose devices and manufacturers – such as Charder - that are already MDR-certified to keep them legal for the market in the future.
What does it mean to be MDR-certified?
Although MDD-certified devices and manufacturers are still valid for now, selecting an MDR-certified company like Charder is highly recommended.
Among other aspects, MDR-certified manufacturers are required to:
- Establish a Risk Management System
- Ensure compliance through a Quality Management System
- Provide EU compliance documentation
- Establish UDI identification system for devices placed on market
- Establish and maintain Post-Market Surveillance System
MDR is needed to access the EU market, but also very useful worldwide. Due to how rigorous the MDR certification process is, many countries will accept MDR as directly equivalent for approval in their own medical device registration systems, or accept MDR-formatted documentation for use in application.
What this means for you:
It is significantly easier to register an MDR-certified device in your own country as a medical device!
Summary
Working with an MDR-certified company like Charder is highly recommended:
- No worry of expiration and being blocked from market
- Safer devices, reducing risk to partners
- Verified Quality Management System for consistent high quality level
- Manufacturer is held accountable by EU authorities
- UDI improves transparency and traceability
- Post-Market Surveillance means manufacturer actively works with you to reduce and fix adverse events
- MDR devices/manufacturers can enter markets more easily
By choosing to distribute MDR-certified devices, you're ensured high-quality products that are safe and effective, compliant with the latest standards used by the EU.